Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy.
Introduction
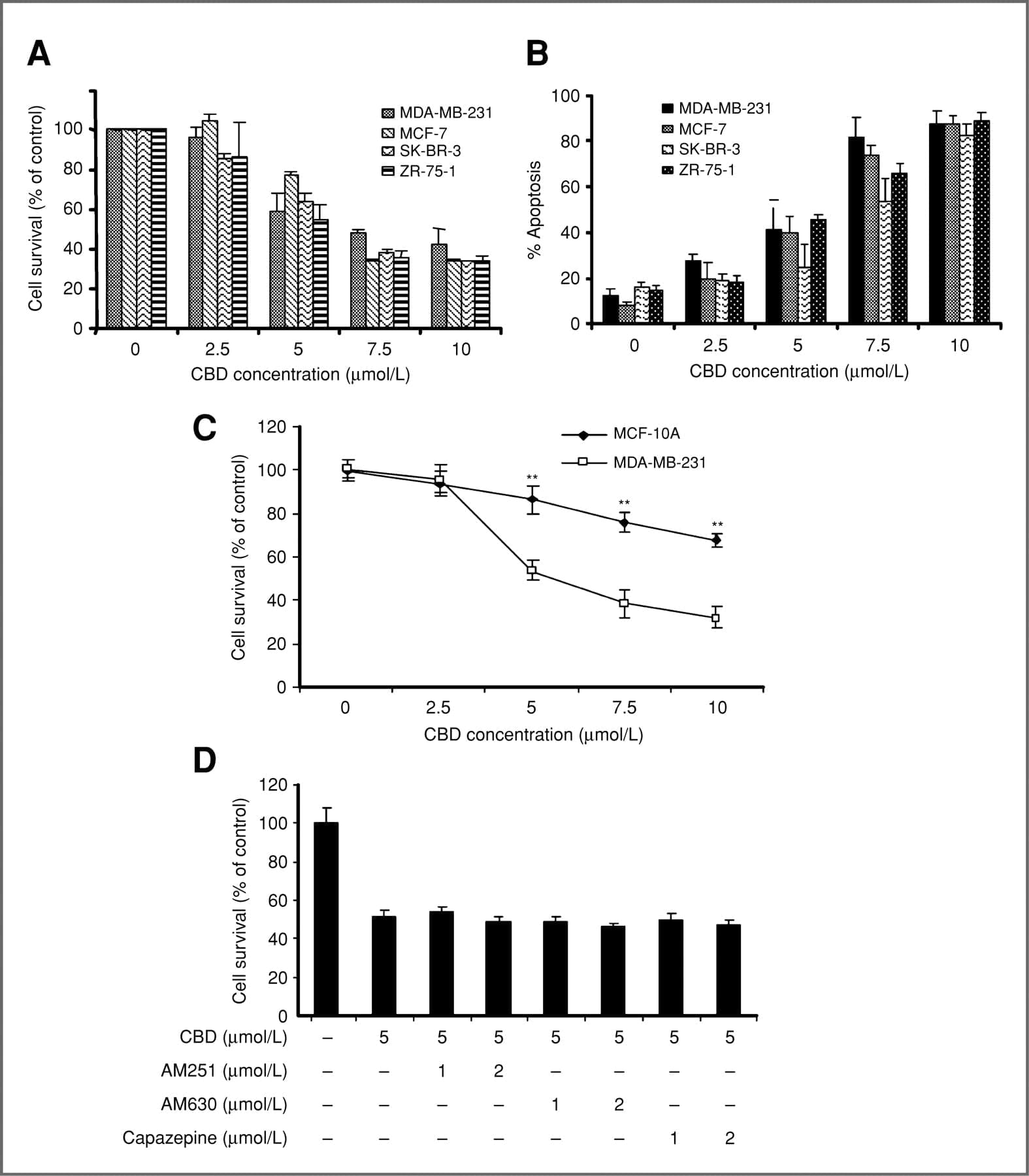
Breast cancer is the second leading cause of cancer-related death in women in the United States (1). Conventional treatment options are often limited by toxicity or acquired resistance, and novel agents are needed. We analyzed the effects of the Cannabis sativa constituent, cannabidiol (CBD), a potent, natural compound with reported activity in breast cancer cell lines, and elucidated its effects on key neoplastic pathways.
CBD belongs to the cannabinoid family, a group of pharmacologically active compounds that bind to specific G-protein–coupled receptors (2). Phytocannabinoids are plant-derived products from Cannabis sativa; endogenous cannabinoids are made in animal and human tissues; and synthetic cannabinoids are laboratory produced. The G-protein–coupled receptor CB1 is found mainly in the brain and nervous system, whereas CB2 is expressed predominantly by immune cells (3). Recent data suggest that some cannabinoids also signal through the vallinoid receptor (4), whereas others may function in a receptor-independent manner (3). Cannabinoids can modulate signaling pathways central to the growth and spread of cancer. They inhibit cell-cycle progression and chemotaxis, and block angiogenesis (5). Recent studies have shown that cannabinoids also induce autophagic cell death (6). Δ9-tetrahydrocannabinol (THC) is one of the best-characterized cannabinoids; however, its therapeutic applications are limited by its psychoactive effects. We focused our work on CBD, a phytocannabinoid devoid of these properties (3).
Although CBD is reportedly effective against various tumors, its molecular mechanism of action is not fully characterized. CBD is cytotoxic to gliomas and inhibits tumor cell migration in vitro (7–9). In addition, CBD induces apoptosis in human leukemia cell lines by activating classical caspase pathways, and enhancing NOX4 and p22 (PHOX) function (10). A recent study reports that CBD inhibits breast cancer growth (11) and downregulates ID1, a regulator of metastasis in breast cancer cell lines (12). Furthermore, CBD, in conjunction with THC, induces programmed cell death (PCD) in glioma cells (13).
PCD, a cell suicide program critical to development and tissue homeostasis, can be classified according to the morphology of a dying cell. Apoptosis is a type I PCD involving caspase activation, phosphotidyl serine inversion and DNA fragmentation (14). More recently, autophagy, a process traditionally considered a survival mechanism, was also implicated as a mode of PCD, when excess de novo–synthesized, double membrane-enclosed vesicles engulf and degrade cellular components (15). The relationship between apoptotic and autophagic death is controversial (16). They may cooperate, coexist, or antagonize each other to balance death versus survival signaling (16). We found that CBD induced both apoptosis and autophagy in breast cancer cells, and evaluated further the effects of CBD on the complex interplay between these 2 types of PCD in breast cancer cell lines. Characterizing more precisely the manner by which CBD kills breast cancer cells will help define the optimal applications of CBD as a cancer therapeutic.
Abstract
Cannabidiol (CBD), a major nonpsychoactive constituent of cannabis, is considered an antineoplastic agent on the basis of its in vitro and in vivo activity against tumor cells. However, the exact molecular mechanism through which CBD mediates this activity is yet to be elucidated. Here, we have shown CBD-induced cell death of breast cancer cells, independent of cannabinoid and vallinoid receptor activation. Electron microscopy revealed morphologies consistent with the coexistence of autophagy and apoptosis. Western blot analysis confirmed these findings. We showed that CBD induces endoplasmic reticulum stress and, subsequently, inhibits AKT and mTOR signaling as shown by decreased levels of phosphorylated mTOR and 4EBP1, and cyclin D1. Analyzing further the cross-talk between the autophagic and apoptotic signaling pathways, we found that beclin1 plays a central role in the induction of CBD-mediated apoptosis in MDA-MB-231 breast cancer cells. Although CBD enhances the interaction between beclin1 and Vps34, it inhibits the association between beclin1 and Bcl-2. In addition, we showed that CBD reduces mitochondrial membrane potential, triggers the translocation of BID to the mitochondria, the release of cytochrome c to the cytosol, and, ultimately, the activation of the intrinsic apoptotic pathway in breast cancer cells. CBD increased the generation of reactive oxygen species (ROS), and ROS inhibition blocked the induction of apoptosis and autophagy. Our study revealed an intricate interplay between apoptosis and autophagy in CBD-treated breast cancer cells and highlighted the value of continued investigation into the potential use of CBD as an antineoplastic agent.
More Resources
Full Text Sources
Medical
Research Materials

 Abstract
Abstract